Research studies
Partners Calciphylaxis Biobank and Patient Registry (PCB)
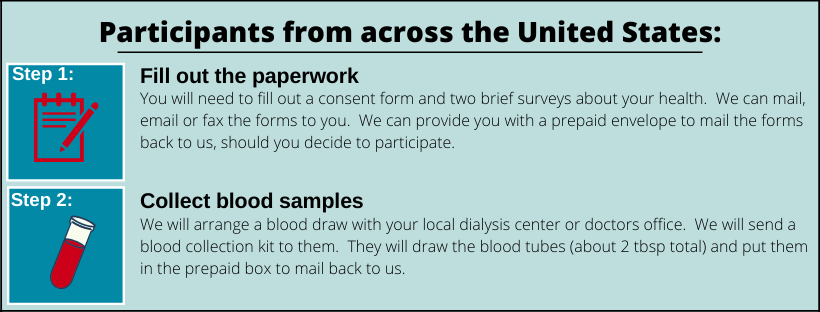
Calciphylaxis is a rare and still very much misunderstood disease. The Partners Calciphylaxis Biobank (PCB) is designed to create a large database of surveys and blood samples to learn more about this terrible disease. If you would like to participate, we would ask you to fill out 2 brief surveys and collect a one time blood draw at your local doctor’s office or dialysis session. You can participate in this study from anywhere in the country. NCT03032835
How the process works:

CALCIPHYX
The CALCIPHYX Study is researching an investigational drug to find out if it can help improve calciphylaxis wound healing and reduce wound pain. The trial is sponsored by Sanifit Therapeutics S.A.. The study consist of a screening period, a double-blind, randomized, placebo-controlled treatment period (Part 1), an open-label treatment period (Part 2), and a follow-up period. During Part 1, participants will receive the study drug or placebo during each of their hemodialysis sessions (3x per week) for twelve weeks. Once Part 1 is completed, all participants will receive the investigational drug during each hemodialysis session (3x per week) over the course of twelve weeks for Part 2. In total, the study duration for each participant will last up to 33 weeks. The sponsor will provide the study drug at no cost to you or the patient. Study participants will be compensated on a pro-rated basis for their participation. NCT04195906
Prior calciphylaxis studies
VitK-CUA
Calcific uremic arteriolopathy a.k.a. calciphylaxis is a vascular calcification disorder seen in dialysis patients. Calcific uremic arteriolopathy has 60-80% one-year mortality and significant morbidity associated with non-healing and extremely painful skin lesions. At present, there is no effective treatment for calcific uremic arteriolopathy. Vitamin K is an important vitamin for inhibiting vascular calcification. It is known to increase the circulating levels of carboxylated Matrix Gla Protein, a potent inhibitor of vascular calcification. However, the effects of vitamin K supplementation in patients with calcific uremic arteriolopathy are unknown. The purpose of this study was to conduct a pilot randomized controlled trial to examine the effects of oral vitamin K supplementation on circulating levels of anti-calcification factor (carboxylated Matrix Gla Protein) and clinical outcomes in patients with calcific uremic arteriolopathy. NCT02278692
CALISTA:
Calciphylaxis is one of the most devastating complications in dialysis patients and at present, there is no FDA approved treatment for calciphylaxis. The CALISTA trial, the first Phase 3 randomized, placebo controlled trial for calciphylaxis, provided an opportunity to understand the efficacy and safety of intravenous sodium thiosulfate in the treatment of hemodialysis patients with calciphylaxis. The trial was sponsored by Hope Pharmaceuticals, a pharmaceutical company based in Scottsdale, AZ. During the trial, participants would receive the study drug or placebo during the last 60 minutes of each hemodialysis session for three weeks. The study duration for each participant lasted up to 4 weeks. The sponsor provided the study drug at no cost to the patient. NCT03319914
Donate to calciphylaxis research here
